Focusing on precision and effectiveness to provide trusted medical writing services for Research, Regulatory, and Publication Needs
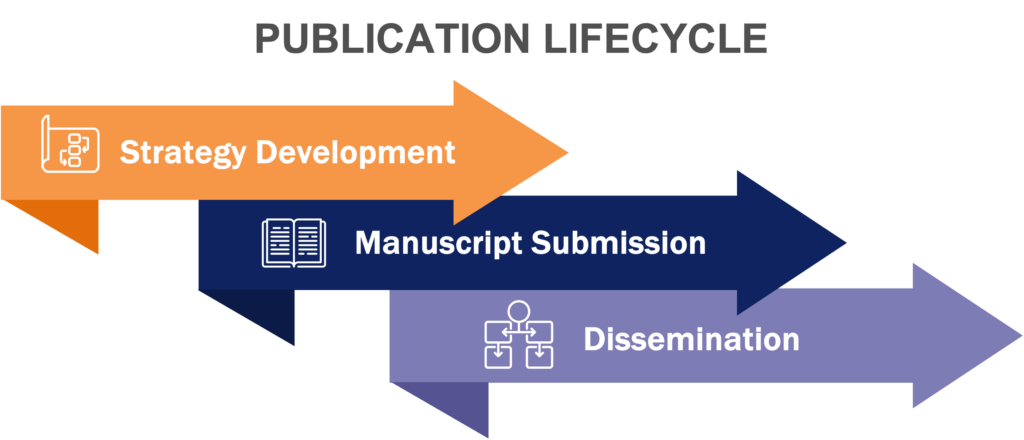
Our team of evidence development experts provides comprehensive clinical, scientific, and HEOR publication planning and development services, ensuring that scientific, economic, and clinical evidence is effectively communicated to the target audience. We manage the entire publication lifecycle, from strategy development to manuscript submission and dissemination.